Alloy Dental Implants
Madris Tomes Kinnard is a former Food and Drug Administration IT project manager. She left the agency because she felt its database did not deliver the adverse event reports in a usable way.
Kinnard then came up with a new database to focus on patient safety. Her business, Device Events, uses the 11 million pieces of data reported to the FDA but makes sense of the adverse events, or complications providing it to anyone who wants to understand how well the 175,000 medical devices in the market are working in the real world.
After they are on the market, tracking devices is useful to patients, doctors, hospitals, journalists, and law firms.
Watching the data is essential for several reasons. First, most medical devices, more than 90 percent, are not approved by the FDA but “cleared” under its 510(k) process. That means the devices can bypass clinical trials required of pharmaceuticals, and with an exchange of paperwork, can be put onto the market in about 90 days or less.
All the device maker has to do is claim that its device is substantially equivalent to a device already on the market, called the predicate device. It is up to the manufacturer to decide just how similar the devices are. With the emergency of new technologies and electronics added to devices, it is unlikely they are substantially equivalent to a device cleared decades ago.
This is a win-win for the manufacturers and not so much for the consumer.
Some of the most dangerous medical devices, later removed from the market or found to be defective, have been cleared through the 510(K), such as hip and knee implants.
Unlike Australia, which has a device event registry monitoring adverse events and looking for trends in complications, the U.S. relies on its MedWatch reporting system and the MAUDE database. Generally, problems are seen in the real world before the FDA notices, such as transvaginal mesh, Essure birth control device, and Metal-on-metal hip prosthesis. Meanwhile, Australia noticed the complications reports coming in. That was the case with metal-on-metal hips (MOM), where Australia’s post-approval monitoring noted a high failure rate with the DePuy ASR implants and recalled them from the market in 2009, one year before a U.S. recall.
Device Events Notes Spikes in Injuries
Not that the FDA collects all data on devices that are harming patients. Ten years ago, the Office of the Inspector General (OIG) estimated only 14 percent of adverse events are ever reported to the FDA.
Still, even with a fraction of the problems ever making it to the agency, Kinard and Device Events have tracked recent spikes in injuries.
For example, there has been a drastic spike in hernia mesh cases reported since 2017. That year there were 3,149 complaints to the FDA. In 2020 that number jumped to 13,942 complaints. Hernia mesh, made of polypropylene, is used in the majority of hernia repairs. Like the mesh used for pelvic organ surgeries, the same polypropylene can migrate, erode, and cause chronic pain and infection.
Hernia mesh has been known to erode into the colon. Often sepsis and death are seen as an outcome of a hernia mesh repair.
With one million hernia surgeries performed annually in the U.S. and doctors relying on mesh rather than an “old fashioned” suture repair, do the math to see the potential for hundreds of thousands of injuries annually.
Device Events has also noted a recent jump in injuries associated with dental implants.
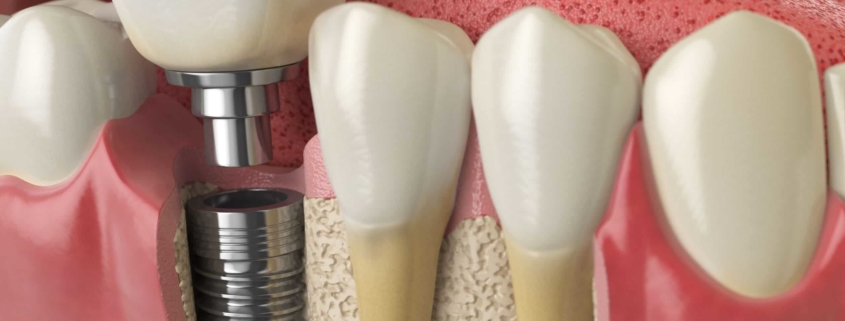
Dental Implants
The FDA received over two million reports on dental implants. Primarily the complication results when the body rejects the dental implant.
The FDA wanted to have more dentists reporting to them about dental implants, and they did a video tutorial on how to report to the FDA about a year and a half ago. Since then, they’ve received 300,000 adverse event reports for dental implants.
According to The International Journal of Implant Dentistry, titanium alloy particles can deposit in surrounding tissue following corrosion and wear. This can lead to bone loss from inflammation or hypersensitive reactions that eventually lead to an implant failure in some patients.
The first step for a patient is to take a diagnostic patch test to determine any metal injuries. An alloy dental implant can be a mixture of titanium with almost any other element. By definition, an alloy is a man-made material.
While most people get titanium, it’s an alloy and can contain other metals such as cobalt and chromium. Those are metals that have caused trouble with metal-on-metal hips.
Even an allergic reaction to titanium alone was noted in one study involving 1,500 dental implant patients.
The FDA considers if your body rejects your dental implant, it is not a malfunction of the device, but it is a severe injury.
Another metal used in the alloys is nickel, a naturally occurring metallic element. With about one-third of the population allergic or sensitive to nickel, patients need to ask what’s in the device they are about to have permanently implanted in their mouth. If you cannot wear earrings with nickel, certainly a dental implant containing it is out of the question.
About ten to 15 percent of the population is allergic to metals, so in considering any permanent implant, it is crucial to know the composition of the implant.
Report Your Injury to the FDA
As imperfect a system as the FDA is in monitoring medical devices after they are released on the market, it is still all that we have at present to notice any spike in injuries. If you or your client has an adverse reaction to a medical device, contact the FDA MedWatch division and report the injury.
Make sure it’s reported correctly and do a follow-up if your condition changes. Keep in touch with the FDA instead of relying on the doctor to do the reporter. Neither the doctor nor the manufacturer has an incentive to report a complication with a device.
###
Sources:
Colgate https://www.colgate.com/en-us/oral-health/implants/titanium-rejection-symptoms-are-you-allergic-to-your-dental-implant
Reuters https://www.reuters.com/article/us-nobelbiocare-lawsuit/dentist-seeks-u-s-class-action-suit-vs-nobel-biocare-idUSTRE6641BV20100705
London Institute of Dentistry https://www.mdpi.com/2673-1592/2/2/11
PubMed https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5633690/
TampaSteel https://tampasteel.com/what-is-a-metal-alloy/
Implant Institute https://implante.institute/blog/can-i-be-allergic-to-titanium-dental-implants/413#:~:text=Virtually%20all%20implants%20are%20alloys,also%20found%20in%20the%20implant.
PubMed https://pubmed.ncbi.nlm.nih.gov/18705814/
https://pubmed.ncbi.nlm.nih.gov/24027897/
The International Journal of Implant Dentistry notes that due to implants‘ corrosion and wear, titanium alloy particles can get deposited in the surrounding tissues. In some people, this can cause bone loss due to inflammatory reactions or hypersensitivity reactions that cause implant failure.
Colgate https://www.colgate.com/en-us/oral-health/implants/titanium-rejection-symptoms-are-you-allergic-to-your-dental-implant
Says first step is to take a diagnostic test such as a patch test to detect metal allergy and the commercially available MELISA test.
National Institutes of health – Allergy related to dental implant and its clinical significance, 2013 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3753052/ Talks about other metals that are part of alloy and may intensify allergic reactions.
Titanium allergy: A Literature Review, 2014 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4248517/
An allergic reaction can be rationally guessed subsequent to metallic implant placement, based on clinical features linked with allergy, such as rash, urticaria, pruritus, oral erythema, swelling in the region, eczematous lesions, or hyperplastic lesions of periodontal tissue (the peri-implant mucosa).Aug 19, 2013
Allergy related to dental implant and its clinical significance
https://www.ncbi.nlm.nih.gov › articles › PMC3753052
https://www.drsirin.com/blog/having-allergic-reaction-to-dental-implants/ there are metal free options.
BioMed Research International Side effects of dental metal implants: Impact of Human Health (Metal as a Risk factor of Implantologic Treatment) 2019 https://www.hindawi.com/journals/bmri/2019/2519205/
Journal of Prosthodontic Research Allergic contact dermatitis caused by titanium screws and dental implants 2015 https://www.sciencedirect.com/science/article/pii/S1883195815001115
A critical review of dental implant materials with an emphasis on titanium versus zirconia, 2015 https://www.researchgate.net/publication/273685161_A_Critical_Review_of_Dental_Implant_Materials_with_an_Emphasis_on_Titanium_versus_Zirconia
LEGAL
THERE IS ALREADY a class action filed against Nobel Biocare of Zurich Switzerland because 8% of implants were lost after implant. US District Court for central California, each member of the class can be reimbursed. Involving thousands of dentists involved in a $1.3 M settlement, May 2013. https://www.massdevice.com/nobel-biocare-settles-450m-class-action-13m/
Legal: Other mistakes with dental implants https://protectingpatientrights.com/blog/dental-implant-mistakes/



Leave a Reply
Want to join the discussion?Feel free to contribute!