Paragard IUD MDL Created in North District of Georgia
It’s an emerging tort that has potential to amass a number of cases.
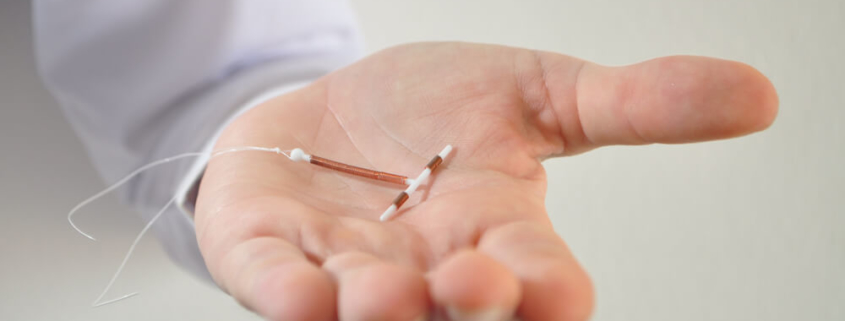
Paragard is an IUD (intrauterine device) used to prevent pregnancy. It was approved by the U.S. Food and Drug Administration (FDA) in 1984.
Paragard is owned by Cooper Surgical, maker of surgical and laparoscopic instruments primarily for women’s surgical needs including devices for hysterectomy, pelvic surgery, and C-section procedures.
Because of the large number of reported complications, multidistrict litigation has recently formed in the North District of Georgia. Currently there are 95 cases filed.
Adverse events reported include the device breakage during explant (removal) surgery causing complications and injuries and the need for follow-up surgery to remove the pieces, infertility, and pain.
Other complications include:
- Organ perforation
- IUD arms embed in uterine wall
- Migration outside of the uterus
- Pieces difficult to locate
- Ectopic pregnancy
- Possible hysterectomy
Copper IUD
IUDs are an increasingly popular form of birth control. There are two types – hormonal and copper.
Paragard is a copper contraceptive that is advertised as “100% hormone free.” Made of flexible polyethylene plastic wrapped with a thin layer of copper coil around the arms and stem, Paragard blocks the sperm from reaching an egg and may also prevent implantation. The copper is emitted and incites an inflammatory reaction toxic to sperm and egg.
Paragard is the only copper emitting IUD used in the U.S. Three other IUDs on the market emit hormones – Mirena, Skyla and Liletta.
About the size of a pack of sugar, the user is not expected to feel Paragard. Periods are expected to be heavier and longer with spotting in between. Pregnancy with an IUD in place is associated with an increased risk of miscarriage, premature labor and delivery, and sepsis. There is even a 27% rate of miscarriage when the IUD is removed from a pregnant woman.
Long-term studies on animals to assess the potential of cancer with a copper-containing IUD have not been performed.
FDA and Paragard
The FDA has warned that Paragard removal may be difficult because of “partial penetration or embedment of Paragard in the myometrium” (middle layer of the uterine wall).
Since it was approved, the FDA has received more than 40,000 reports of complications associated with Paragard including 15 deaths, however, Paragard remains on the market.
In an FDA letter to CooperSurgical Regulatory Affairs, dated July 2019, the agency warns the direct-to-consumer (DTC) advertising with the dancing Paragard users, makes false or misleading representations about the risks associated with Paragard.
IUDs have long been associated with an increase in pelvic inflammatory disease (PID), infertility and death.
The use of an IUD as a reliable form of birth control has been around for decades but IUDs gained a bad reputation from the complications associated with use of the Dalkon Shield of the 1970s. Hundreds of thousands of women reported problems with pelvic inflammatory disease, infertility, spontaneous abortions, and death. The Dalkon Shield incident encouraged the passage of the Medical Device Amendments of 1976, that, for the first time, gave the FDA some control over medical devices.
In another case of IUD complications, Bayer offered a $12.2 million settlement in 2018 to thousands of claimants over complications associated with Mirena IUD.
ParaGard MDL
On December 16, 2020, the Judicial Panel on Multidistrict Litigation (JPML) issued a transfer order creating the Paragard MDL. So far, 95 cases have been filed in MDL 2974 in the Northern District of Georgia before U.S. District Judge Leigh Martin May. At the time, there were 80 actions pending in 36 districts.
The defective product action will include manufacturing defect, defective design, marketing, promotion, labeling, packaging, and distribution, and a failure-to-warn, gross negligence, and consumer protection law fraud. The plaintiffs seek punitive damages.
The defendants are alleged to have understood the risks from clinical trials and post-marketing complaints but failed to warn and instead concealed and suppressed the dangers, one complaint reads. (Plendl v Teva et al)
CooperSurgical opposed the centralization of cases but the JPLM rejected claims that the creation of an MDL would generate the filing of voluminous claims without due diligence by lawyers for the plaintiffs. Instead, it found the Northern District of Georgia serviced the convenience of the parties involved. Six of the actions were already filed in the Northern District of Georgia. None of the cases has been resolved.
The new drug application (NDA) for Paragard was previously held by Teva Women’s Health, Inc. from November 10, 1995 to August 11, 2017. Most of the actions filed so far name Teva Pharmaceuticals USA, Inc. et al.
Previously, Duramed Pharmaceuticals, Inc., a division of Barr Pharmaceuticals Inc. DBA Teva Women’s Health Inc., acquired FEI (Finishing Enterprises Inc.) Women’s Health in 2005. The deal included the Paragard IUD.
Duramed was acquired by Teva USA in 2008. Cooper Companies, a Delaware Corporation with headquarters in Pleasanton, California, purchased assets of Paragard IUD in September 2017 for $1.1 billion. CooperSurgical is a subsidiary of Cooper Companies. Under Georgia law, successors-in-interest are liable.
Sources:
Pending mdl https://www.jpml.uscourts.gov/sites/jpml/files/Pending_MDL_Dockets_By_District-January-15-2021.pdf
FDA letter
https://www.fda.gov/media/129526/download



Leave a Reply
Want to join the discussion?Feel free to contribute!